Examples:
|
Eye Glasses |
Hands |
Chair |
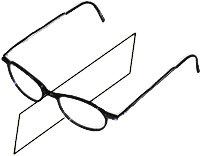
Look at a pair of glasses and see the reflection symmetry from the center. There is no other symmetry.
|

Look at your hands to see reflection symmetry. Can you see any more symmetry operations?
|
|
Back to top
H20 |
|
|
|
Mirror planes must either bisect the molecule or contain all atoms in a molecules. The mirror planes for H20are examples of each of these situations. |
Back to top
CO2 |
|
|
| Is it hard to see infinite number of reflection planes? All of those planes contain all three atoms. |
Back to top
Benzene |
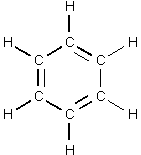
How many planes can you see? |
|
If you said 7 for the above question, you are on the right track. Guess which one is the seventh. For hint, check the water example above. Back to top |



